Abstract
Introduction
In CLL, progressive disease (PD) following remission after first line treatment with chemoimmunotherapy can present with varying clinical phenotypes. According to iwCLL guidelines, criteria of PD can be lymphocytosis, lymphadenopathy, organomegaly, cytopenia, or disease transformation. We hypothesized that mode of PD correlates with different clinical outcomes following initial remission upon first line therapy of CLL.
Methods
Data from three phase III trials of the GCLLSG (CLL8, CLL10, CLL11) including a total of 2159 patients (pts) receiving first line therapy were analysed. Fit pts from the CLL8 and CLL10 studies receiving fludarabine and cyclophosphamide (FC), FC and rituximab (FCR) or bendamustine and R (BR) were pooled to a cohort of 1378 pts. 781 unfit pts from the CLL11 study receiving chlorambucil (CLB), CLB-R or CLB-obinutuzumab (CLB-Ob) were analysed similarly. Laboratory, genetic, event-related, and health-related quality of life (HRQoL) data were pooled. Pts were categorized as "ALC" if PD was due to increasing absolute lymphocyte count, or as "Ly" if due to progressive lymphadenopathy. Kaplan-Meier curves for time-to-next treatment (TTNT) and overall survival (OS) after PD were plotted and compared by non-stratified log-rank test. Hazard ratios (HR) and 95% confidence intervals were calculated using Cox regression modelling.
Results
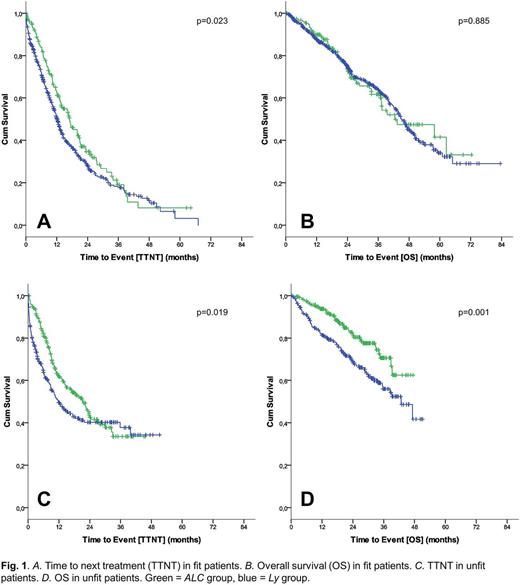
425 pts from CLL8 (FCR vs FC) and 251 pts from CLL10 (FCR vs BR) with data available for PD categorization were pooled to a cohort of 676 pts. 171 (25%) pts solely progressed with lymphocytes (ALC) and 505 (75%) pts progressed with lymphadenopathy (Ly), including 201 (30%) pts progressing with both lymphocytes and lymph nodes. Except for del11q, which was more frequent in the Ly group (33% vs 26%), no differences in the characteristics, including distribution of CLL-IPI risk groups, were observed.
Likewise, 463 unfit pts with available PD data from the CLL11 trial were analysed separately. 241 (52%) pts progressed with ALC and 222 (48%) pts progressed with Ly, including 128 (28%) pts progressing with lymphocytes and lymph nodes. Trisomy 12 was more frequent in the Ly group (23% vs 12%), while del13q was more frequent in the ALC group (59% vs 43%). Otherwise, no differences in the characteristics, including CLL-IPI risk groups, were observed between the ALC and Ly group.
After occurrence of PD in pts treated with FC/FCR/BR, median TTNT in the Ly group was 12.3 months vs 17.0 months in the ALC group (HR 1.299 [1.036-1.628]; p=0.024). Median OS was 45.1 months in the Ly group and 42.4 months in the ALC group (HR=1.023 [0.753-1.389]; p=0.885). For pts receiving CLB-based therapy, median TTNT in the Ly group was 11.7 months vs 21.4 months in the ALC group (HR 1.357 [1.051-1.753]; p=0.019). Median OS was 42.8 months in the Ly group and not reached in the ALC group (HR 1.851 [1.280-2.677]; p=0.001).
Fit pts in the Ly group more frequently showed B-symptoms (11% vs 4%) and signs of bone marrow failure (32% vs 20%) at the time of PD as compared to the ALC group. Mean absolute HRQoL scores in the Ly group showed impaired physical (80 vs 88) and emotional (72 vs 80) functioning, more fatigue (36 vs 26), dyspnoea (26 vs 14) and reduced global health status (67 vs 73) at time of PD. Similar figures were observed in unfit patients.
Discussion
This analysis demonstrates that the mode of progression after first line therapy of CLL could affect further outcome, with progressive lymphadenopathy being associated with a significantly shorter TTNT, including a shorter OS in unfit pts, and a less favourable clinical profile as compared to progression by lymphocytosis. These data might help physicians to better estimate TTNT, OS and the clinical course of a progressing CLL patient.
Al-Sawaf: Gilead: Other: Travel fees; Roche: Honoraria, Other: Travel fees; Abbvie: Honoraria, Other: Travel fees. Bazeos: Roche: Other: • A year-long academic collaboration contract with Roche (no financial gain).. Bahlo: F. Hoffmann-LaRoche: Honoraria, Other: travel grants. Fink: Hoffmann La Roche: Other: Travel grant; Celgene: Other: Grant; Mindipharma: Other: Travel Grant. Von Tresckow: Roche: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria; Janssen: Honoraria, Research Funding. Cramer: F. Hoffmann-LaRoche: Honoraria, Other: travel support, Research Funding; Gilead: Other: travel support, Research Funding; AbbVie: Consultancy; Janssen-Cilag: Consultancy, Honoraria, Other: travel support, Research Funding; GSK: Research Funding; Novartis: Consultancy, Research Funding; AstraZeneca: Consultancy. Langerbeins: Janssen-Cilag: Consultancy, Honoraria, Research Funding; Abbvie: Honoraria; F. Hoffmann-LaRoche: Consultancy, Honoraria; Mundipharma: Other: Travel support. Humphrey: Roche: Employment. Fingerle-Rowson: F. Hoffmann-La Roche: Employment. Stilgenbauer: Gilead: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; Genzyme: Consultancy, Honoraria, Research Funding; Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Boehringer-Ingelheim: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria, Research Funding; Mundipharma: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; GSK: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding. Wendtner: Abbvie: Other: Personal Fees; Morphosys: Other: Personal Fees; Servier: Other: Grant, Personal Fees; Gilead: Other: Personal Fees; Novartis: Other: Personal Fees; Janssen-Cilag: Other: Personal Fees; Mundipharma: Other: Grant, Personal Fees; Celgene: Consultancy, Research Funding; Hoffmann La Roche: Other: Grant, Personal Fees. Fischer: Roche: Other: Travel Grants. Eichhorst: Janssen-Cilag: Consultancy, Honoraria, Other: travel support, Research Funding; Gilead: Consultancy, Honoraria, Other: travel support, Research Funding; F. Hoffmann-LaRoche: Consultancy, Honoraria, Other: tra, Research Funding; Abbvie: Consultancy, Honoraria, Other: trav, Research Funding; Mundipharma: Consultancy, Honoraria, Other: travel support, Research Funding; GlaxoSmithKline: Consultancy, Honoraria, Other: travel support, Research Funding; Celgene: Consultancy, Honoraria, Other: travel support, Research Funding. Hallek: Abbvie: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding, Speakers Bureau. Goede: Gilead: Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel grants, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel grants, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal